Chemistry, 28.09.2019 01:30 daltonrebekah3532
Propane can be turned into hydrogen by the two-step reforming process. in the first step, propane and water react to form carbon monoxide and hydrogen: c3hg(g)+3 3 co(g)+7 h2(g) in the second step, carbon monoxide and water react to form hydrogen and carbon dioxide: co(g)+h20(g)h2(9)+co2(g) write the net chemical equation for the production of hydrogen from propane and water. be sure your equation is balanced.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

You know the right answer?
Propane can be turned into hydrogen by the two-step reforming process. in the first step, propane an...
Questions

Mathematics, 10.12.2019 11:31


Mathematics, 10.12.2019 11:31

History, 10.12.2019 11:31

Mathematics, 10.12.2019 11:31

Mathematics, 10.12.2019 11:31

Mathematics, 10.12.2019 11:31





Mathematics, 10.12.2019 11:31


Mathematics, 10.12.2019 11:31






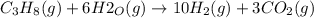
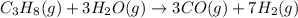 ...[1]
...[1]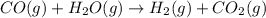 ...[2]
...[2]