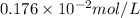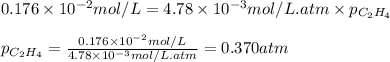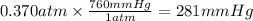Chemistry, 28.09.2019 01:30 MIAkwicc39
What partial pressure of c2h4 gas (in mm hg) is required to maintain a solubility of 4.92×10-2 g/l in water at 25 °c? kh for c2h4 at 25 °c is 4.78×10-3 mol/l·atm.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1
You know the right answer?
What partial pressure of c2h4 gas (in mm hg) is required to maintain a solubility of 4.92×10-2 g/l i...
Questions



Health, 17.07.2019 10:00


Mathematics, 17.07.2019 10:00


Social Studies, 17.07.2019 10:00


Social Studies, 17.07.2019 10:00


Social Studies, 17.07.2019 10:00

Social Studies, 17.07.2019 10:00


Social Studies, 17.07.2019 10:00

Social Studies, 17.07.2019 10:00

Mathematics, 17.07.2019 10:00


Computers and Technology, 17.07.2019 10:00

Social Studies, 17.07.2019 10:00

Social Studies, 17.07.2019 10:00

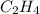 is 281 mmHg
is 281 mmHg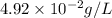
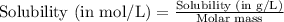
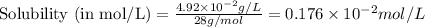
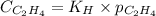
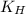 = Henry's constant =
= Henry's constant = 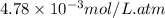
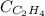 = molar solubility of ethene gas =
= molar solubility of ethene gas =