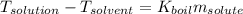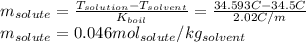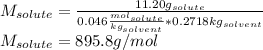The boiling point of diethyl ether, ch3ch2och2ch3, is 34.500 °c at 1 atmosphere. kb(diethyl ether) = 2.02 °c/m
in a laboratory experiment, students synthesized a new compound and found that when 11.20 grams of the compound were dissolved in 271.8 grams ofdiethyl ether, the solution began to boil at 34.593 °c. the compound was also found to be nonvolatile and a non-electrolyte.
what is the molecular weight they determined for this compound ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
The boiling point of diethyl ether, ch3ch2och2ch3, is 34.500 °c at 1 atmosphere. kb(diethyl ether) =...
Questions


History, 28.07.2019 10:30





Social Studies, 28.07.2019 10:30

English, 28.07.2019 10:30




History, 28.07.2019 10:30






Mathematics, 28.07.2019 10:30