
Chemistry, 28.09.2019 02:10 Sylnatius6736
4. al2o3 (s) + 6hcl (aq) → 2alcl3 (aq) + 3h20(1) find the mass of alcl3 that is produced when 10.0 grams of al2o3 react with 10.0 g of hci moar mass alqo3 102 gm imol al2o3 = 0.098 moles molar mass of hcl = 36, 5gr/mol #of moles #6l =0,274 moles mole of al2 oz 6 mol of hc 01274 x2 = 0.0913 moles alc3=13,5 6 i mass alco3 = 12, 193gm. 5. how many grams of the excess reagent in question 4 are left over?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1


Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
4. al2o3 (s) + 6hcl (aq) → 2alcl3 (aq) + 3h20(1) find the mass of alcl3 that is produced when 10.0 g...
Questions





Mathematics, 18.08.2021 16:40


English, 18.08.2021 16:40

Physics, 18.08.2021 16:40


Mathematics, 18.08.2021 16:40



Computers and Technology, 18.08.2021 16:40

Chemistry, 18.08.2021 16:40

Mathematics, 18.08.2021 16:40

Mathematics, 18.08.2021 16:40

Mathematics, 18.08.2021 16:40


Computers and Technology, 18.08.2021 16:40

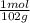 = 0,0980 moles
= 0,0980 moles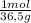 = 0,274 moles
= 0,274 moles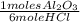 = 0,0457 moles of Al₂O₃
= 0,0457 moles of Al₂O₃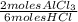 × 133
× 133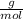 = 12,1 g of AlCl₃
= 12,1 g of AlCl₃ = 5,33 g of Al₂O₃
= 5,33 g of Al₂O₃ 

