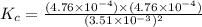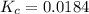Gaseous hydrogen iodide is placed in a closed container at 425°c, where it partially decomposes to hydrogen and iodine: 2hi(g)⇌h₂(g)+i₂(g) at equilibrium it is found that [hi]= 3.51×10⁻³ m, [h₂]= 4.76×10⁻⁴ m, and [i₂]= 4.76×10⁻⁴ m.
what is the value of  at this temperature? express the equilibrium constant to three significant digits.
at this temperature? express the equilibrium constant to three significant digits.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Gaseous hydrogen iodide is placed in a closed container at 425°c, where it partially decomposes to h...
Questions






Advanced Placement (AP), 29.01.2021 14:00

Spanish, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00

English, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00

Computers and Technology, 29.01.2021 14:00


Biology, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00


Social Studies, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00

Biology, 29.01.2021 14:00

Geography, 29.01.2021 14:00

Mathematics, 29.01.2021 14:00

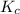 at this temperature is 0.0184
at this temperature is 0.0184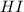 at equilibrium =
at equilibrium = 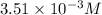
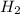 at equilibrium =
at equilibrium = 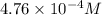
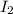 at equilibrium =
at equilibrium = 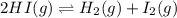
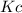 will be,
will be,![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0269/8146/ef85e.png)