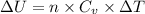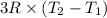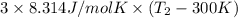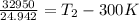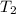Chemistry, 28.09.2019 02:20 IsabellaGracie
Two moles of ideal he gas are contained at a pressure of 1 atm and a temperature of 300 k. 34166 j of heat are transferred to the gas, as a result of which the gas expands and does 1216 j of work against its surroundings. the process is reversible. (note: c = 1.5r) calculate the final temperature of the gas

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2

Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
Two moles of ideal he gas are contained at a pressure of 1 atm and a temperature of 300 k. 34166 j o...
Questions



History, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01

Mathematics, 16.10.2020 06:01



Chemistry, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01

History, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01



English, 16.10.2020 07:01

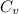 =
= 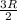
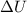 ) = Q + W
) = Q + W