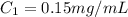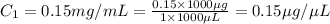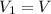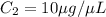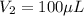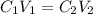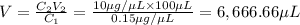You are given a protein solution with a concentration of 0.15 mg/ml.
v. suppose that we...

Chemistry, 28.09.2019 02:20 Pandorasbx2657
You are given a protein solution with a concentration of 0.15 mg/ml.
v. suppose that we want to prepare 100 microliters of 10 micrograms/microliters solution. how much of h2o and protein stock do we need to add to obtain the target concentration and volume? (the concentration is not greater in the question, you need to convert it to micrograms/ then it should make sense)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Questions


English, 16.01.2020 00:31



Chemistry, 16.01.2020 00:31



English, 16.01.2020 00:31



Mathematics, 16.01.2020 00:31



Spanish, 16.01.2020 00:31


Mathematics, 16.01.2020 00:31




Computers and Technology, 16.01.2020 00:31