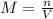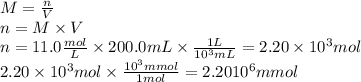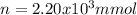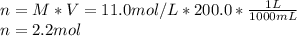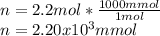Chemistry, 28.09.2019 02:30 michellegregg10
Achemist adds 200.0 ml of a 11.0m silver perchlorate (agcio solution to a reaction flask. calculate the millimoles of silver perchlorate the chemist has added to the flask. be sure your answer has the correct number of significant digits. mmol op ?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Achemist adds 200.0 ml of a 11.0m silver perchlorate (agcio solution to a reaction flask. calculate...
Questions

Business, 22.04.2021 19:00



Mathematics, 22.04.2021 19:00


History, 22.04.2021 19:00


Health, 22.04.2021 19:00





Mathematics, 22.04.2021 19:00

Mathematics, 22.04.2021 19:00

Mathematics, 22.04.2021 19:00


History, 22.04.2021 19:00