
Chemistry, 28.09.2019 03:10 Tonyang1742
Calculate as° for the reaction below: n2(g)+202(g) 2no2(g) where aso for n2(g), o2(g), & no2(g), respectively, is 191.5, 205.0, & 240.5 j/mol-k -156.0 j/k 156.0 j/k 120.5 j/k -120.5 j/k o ooo

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
Calculate as° for the reaction below: n2(g)+202(g) 2no2(g) where aso for n2(g), o2(g), & no2(g...
Questions

Physics, 31.05.2021 14:00

Business, 31.05.2021 14:00

English, 31.05.2021 14:00

Mathematics, 31.05.2021 14:00

Chemistry, 31.05.2021 14:00

Social Studies, 31.05.2021 14:00

Social Studies, 31.05.2021 14:00

Physics, 31.05.2021 14:00



Business, 31.05.2021 14:00

Computers and Technology, 31.05.2021 14:00


Mathematics, 31.05.2021 14:00

Physics, 31.05.2021 14:00

Health, 31.05.2021 14:00

English, 31.05.2021 14:00

Mathematics, 31.05.2021 14:00


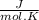 - [ 1 mol × 191,5
- [ 1 mol × 191,5 


