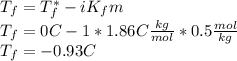Calculate the freezing temperature of the following solution of 0.50 m glucose (a covalent compound). assume that the molality of the solution is 0.50 m. (the molar and molal concentrations of dilute aqueous solutions are often identical to two significant figures.) enter your answer in the provided box. 0.50 m glucose (a covalent compound) °c

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Calculate the freezing temperature of the following solution of 0.50 m glucose (a covalent compound)...
Questions

Mathematics, 27.12.2020 01:00

Mathematics, 27.12.2020 01:00




English, 27.12.2020 01:00

Mathematics, 27.12.2020 01:00

Mathematics, 27.12.2020 01:00

Mathematics, 27.12.2020 01:00







Mathematics, 27.12.2020 01:00

Business, 27.12.2020 01:00


Social Studies, 27.12.2020 01:00

Biology, 27.12.2020 01:00

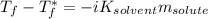
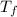 is the freezing temperature of the solution,
is the freezing temperature of the solution,  is the freezing temperature of the pure solvent (0 °C since it is water),
is the freezing temperature of the pure solvent (0 °C since it is water),  the Van't Hoff factor (1 since the solute is covalent),
the Van't Hoff factor (1 since the solute is covalent), 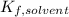 the solvent's freezing point depression point constant (in this case
the solvent's freezing point depression point constant (in this case  ) and
) and 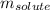 the molality of the glucose.
the molality of the glucose.