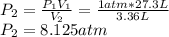Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Calculate the pressure, in atmospheres, required to compress a sample of helium gas from 27.3 l (at...
Questions


Business, 18.07.2019 10:30



Mathematics, 18.07.2019 10:30


Mathematics, 18.07.2019 10:30




Mathematics, 18.07.2019 10:30

Mathematics, 18.07.2019 10:30



Social Studies, 18.07.2019 10:30

Advanced Placement (AP), 18.07.2019 10:30

English, 18.07.2019 10:30

Mathematics, 18.07.2019 10:30


Mathematics, 18.07.2019 10:30

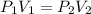
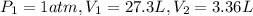
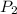 :
: