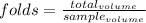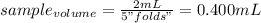Chemistry, 28.09.2019 03:30 Kareemgreen1237
What volume of sample, in ml, would be needed to make 2 ml of a 5-fold dilution? (3 significant figures needed)

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:04
1. according to the article, the chinese government has tried to control its people in all of the following ways except: question 1 options: create and spread propaganda in the media censorship of the media allowing people to create their own social media deleting people's social media posts without their permission question 2 (1 point) 2. when a government has total control over its citizens, including the media, it is a of government question 2 options: constitutional monarchy parliamentary democracy autocracy presidential democracy question 3 (1 point) according to the article, "social credit" is question 3 options: an experiment to people talk on-line a farming experiment that hurt people under mao ze dong using someone else's data from the internet, to give or take away things such as the right to buy an airline ticket a credit you get in china for having friends in real life, and not just on social media question 4 (1 point) china wants to take control over social media in which other region? question 4 options: taiwan chile japan hong kong
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
What volume of sample, in ml, would be needed to make 2 ml of a 5-fold dilution? (3 significant fig...
Questions

Mathematics, 17.09.2019 01:30



English, 17.09.2019 01:30


Business, 17.09.2019 01:30






Social Studies, 17.09.2019 01:30

Biology, 17.09.2019 01:30


Mathematics, 17.09.2019 01:30

Chemistry, 17.09.2019 01:30

Biology, 17.09.2019 01:30

Mathematics, 17.09.2019 01:30

Physics, 17.09.2019 01:30

Mathematics, 17.09.2019 01:30