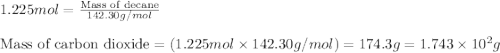Chemistry, 28.09.2019 03:30 kyramks421
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alkane. termed hydrogenation, this type of reaction is used to produce products such as margarine. a typical hydrogenation reaction is c10h20() + h2(g) → c10h22(5) decene decane how much decane can be produced in a reaction of excess decene with 2.45 g hydrogen? give your answer in scientific notation. o *10 g decane

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alk...
Questions

Mathematics, 25.11.2021 15:30




Mathematics, 25.11.2021 15:30



Geography, 25.11.2021 15:30

Arts, 25.11.2021 15:30



Mathematics, 25.11.2021 15:30

Business, 25.11.2021 15:30

Mathematics, 25.11.2021 15:30

English, 25.11.2021 15:30

Mathematics, 25.11.2021 15:30




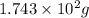
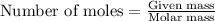 ......(1)
......(1)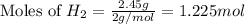
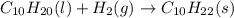
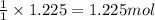 of decane
of decane