
Chemistry, 28.09.2019 05:30 negritoboy014
One container of tumsr costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tumsr is 40.0 percent caco₃ by mass. using only tumsr, you are required to neutralize 0.500 l of 0.400 m hcl. how much does this cost? assume you are able to purchase individual tablets. express your answer in dollars.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 23.06.2019 02:40
How can a mixture of salt water be separated into salt and water
Answers: 1

Chemistry, 23.06.2019 04:00
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
You know the right answer?
One container of tumsr costs 4.00 dollars. each container has eighty 1.00 g tablets. assume each tum...
Questions

Mathematics, 04.06.2021 15:50




Mathematics, 04.06.2021 15:50

English, 04.06.2021 15:50

Mathematics, 04.06.2021 15:50

English, 04.06.2021 15:50

Mathematics, 04.06.2021 15:50

Mathematics, 04.06.2021 15:50

Chemistry, 04.06.2021 15:50


History, 04.06.2021 15:50

Mathematics, 04.06.2021 15:50



French, 04.06.2021 15:50


Mathematics, 04.06.2021 15:50

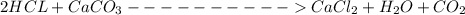
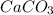 and HCl is 1 : 2
and HCl is 1 : 2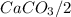 mol HCl = 0.1 mol
mol HCl = 0.1 mol 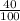 = 0.4
= 0.4 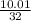 = 0.3128125 (number of containers)
= 0.3128125 (number of containers)

