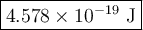Chemistry, 28.09.2019 23:10 zacksoccer8279
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission spectrum has a violet, a blue, a teal, and a red line. calculate the energy of one photon of this light.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1
You know the right answer?
The blue line of the hydrogen emission spectrum has a wavelength of 433.9 nm. a hydrogen emission sp...
Questions




English, 18.03.2021 03:30





English, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30







Mathematics, 18.03.2021 03:30

Social Studies, 18.03.2021 03:30