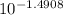Chemistry, 30.09.2019 21:00 geunagillis1
Abuffered solution containing dissolved aniline, c6h5nh2, and aniline hydrochloride, c6h5nh3cl, has a ph of 5.75.
a) determine the concentration of c6h5nh3+ in the solution if the concentration of c6h5nh2 is 0.245 m. the pkb of aniline is 9.13.
[c6h5nh3+]= m
b) calculate the change in ph of the solution, \delta ph, if 0.360g naoh is added to the buffer for a final volume of 1.50l.
\deltaph=

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1



Chemistry, 23.06.2019 10:50
Achemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. the chemical reaction that occurred is shown. na + cl2 → nacl if the percentage yield of the reaction is 86%, what is the actual yield? show your work, including the use of stoichiometric calculations and conversion factors.
Answers: 1
You know the right answer?
Abuffered solution containing dissolved aniline, c6h5nh2, and aniline hydrochloride, c6h5nh3cl, has...
Questions


Chemistry, 31.10.2019 05:31

History, 31.10.2019 05:31

History, 31.10.2019 05:31


Mathematics, 31.10.2019 05:31


History, 31.10.2019 05:31

Mathematics, 31.10.2019 05:31







Chemistry, 31.10.2019 05:31




English, 31.10.2019 05:31

![pH = pKa + log\frac{[A^-]}{[HA]}](/tpl/images/0277/5492/19c19.png)