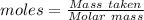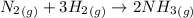Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g) → 2nh3(g) calculate the mass of ammonia produced when 37.0 g of nitrogen react with 12.0 g of hydrogen. g nh3 which is the excess reactant and how much of it will be left over when the reaction is complete? hydrogen nitrogen

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2


Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2
You know the right answer?
Ammonia is produced by the reaction of nitrogen and hydrogen according to the equation n2(g) + 3h2(g...
Questions



Mathematics, 04.12.2020 19:40


Mathematics, 04.12.2020 19:40


Mathematics, 04.12.2020 19:40

Mathematics, 04.12.2020 19:40



Chemistry, 04.12.2020 19:40

Geography, 04.12.2020 19:40



Arts, 04.12.2020 19:40

Social Studies, 04.12.2020 19:40


Mathematics, 04.12.2020 19:40


Mathematics, 04.12.2020 19:40

 .
.