
Astudent determines the molar mass of a compound by the method used in this experiment. she found the temperature of the ice water to be 1.0°c. when she added 11.1g of her unknown solute to the mixture, the temperature fell to -3.0°c. the mass of the solution was 90.4g. calculate mass of solute in solution 1kg of water

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Astudent determines the molar mass of a compound by the method used in this experiment. she found th...
Questions


Mathematics, 22.01.2020 04:31

Mathematics, 22.01.2020 04:31

Mathematics, 22.01.2020 04:31

Mathematics, 22.01.2020 04:31



Biology, 22.01.2020 04:31

Mathematics, 22.01.2020 04:31

Biology, 22.01.2020 04:31

Social Studies, 22.01.2020 04:31


English, 22.01.2020 04:31

Social Studies, 22.01.2020 04:31




Biology, 22.01.2020 04:31


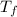 =-3.0°C
=-3.0°C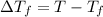
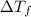 =1.0°C-( -3.0°C)=4°C
=1.0°C-( -3.0°C)=4°C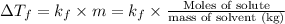
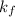 = Molal depression constant
= Molal depression constant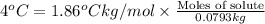
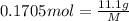
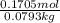
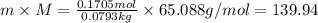 g of solute /kg of water
g of solute /kg of water

