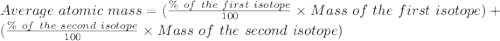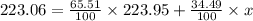Chemistry, 01.10.2019 00:30 20guadalupee73248
Afictitious element z has an average atomic mass of 223.06 u.223.06 u. element z has two naturally occuring isotopes. the more abundant isotope has an exact mass of 223.95 u223.95 u and a relative abundance of 65.51%.65.51%. calculate the exact mass of the second isotope.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 18:50
At stp, which substance is the best conductor of electricity? a. nitrogen b. neon c. sulfur d. silver
Answers: 1
You know the right answer?
Afictitious element z has an average atomic mass of 223.06 u.223.06 u. element z has two naturally o...
Questions




Mathematics, 24.07.2020 16:01


Physics, 24.07.2020 16:01

Law, 24.07.2020 16:01


Mathematics, 24.07.2020 16:01


Spanish, 24.07.2020 16:01

Mathematics, 24.07.2020 16:01

Mathematics, 24.07.2020 16:01



Mathematics, 24.07.2020 16:01




English, 24.07.2020 16:01