
Chemistry, 01.10.2019 03:30 henryzx900
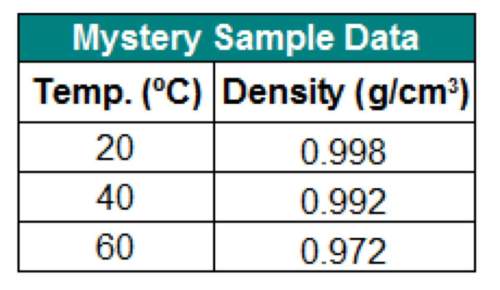
The table below shows the density of a sample of a mystery liquid you tested in the lab. can you infer the identity of the substance from these data?
a. yes, the substance must be water.
b. no, more data are needed.
c. no, the data must be wrong because density always decreases with an increase in temperature.
d. yes, but only if the data for 50ºc and 70ºc were also present.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
The table below shows the density of a sample of a mystery liquid you tested in the lab. can you inf...
Questions


History, 02.09.2019 04:00





Mathematics, 02.09.2019 04:00

English, 02.09.2019 04:00




English, 02.09.2019 04:00

Mathematics, 02.09.2019 04:00


Mathematics, 02.09.2019 04:00

Mathematics, 02.09.2019 04:00

English, 02.09.2019 04:00

Advanced Placement (AP), 02.09.2019 04:00

Mathematics, 02.09.2019 04:00



