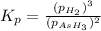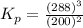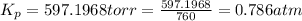Chemistry, 01.10.2019 04:20 gadgetady5699
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 h the pressure in the flask was observed to be constant at 488.0 torr. a. calculate the equilibrium pressure of h2(g). b. calculate kp for this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment p...
Questions

Mathematics, 03.12.2020 22:50


Mathematics, 03.12.2020 22:50






Mathematics, 03.12.2020 22:50


Mathematics, 03.12.2020 22:50

Mathematics, 03.12.2020 22:50





SAT, 03.12.2020 22:50

English, 03.12.2020 22:50


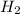 gas is, 288 torr
gas is, 288 torr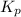 for this reaction is, 0.786 atm
for this reaction is, 0.786 atm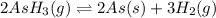
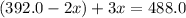
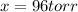
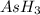 at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr
at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr