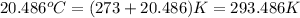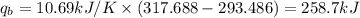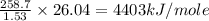Chemistry, 01.10.2019 17:20 mauifrifer3986
Oxyacetylene torches produce such high temperature that they are often used to weld and cut metal. when 1.53 g of acetylene (c2h2) is burned in a bomb calorimeter with a heat capacity of 10.69 kj/k, the temperature increases from 20.486°c to 44.688°c. what is δe (in kj/mol) for this combustion reaction? enter to 0 decimal places.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 23.06.2019 13:20
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer. suppose a chemical engineer studying a new catalyst for the haber reaction finds that 671 liters per second of dinitrogen are consumed when the reaction is run at 271c and 0.99atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. round your answer to significant digits.
Answers: 3
You know the right answer?
Oxyacetylene torches produce such high temperature that they are often used to weld and cut metal. w...
Questions



Social Studies, 18.11.2019 03:31

Health, 18.11.2019 03:31

Mathematics, 18.11.2019 03:31



Biology, 18.11.2019 03:31

English, 18.11.2019 03:31



Physics, 18.11.2019 03:31

Mathematics, 18.11.2019 03:31





Mathematics, 18.11.2019 03:31


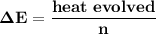
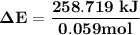
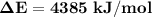
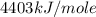
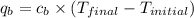
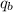 = heat absorbed by calorimeter = ?
= heat absorbed by calorimeter = ?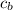 = specific heat of = 10.69 kJ/K
= specific heat of = 10.69 kJ/K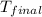 = final temperature =
= final temperature = 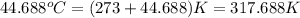
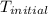 = initial temperature =
= initial temperature =