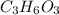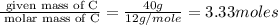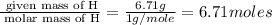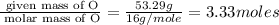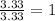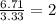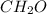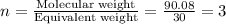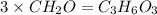Chemistry, 01.10.2019 17:30 MansellS5529
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in carbon, 6.71% mass in hydrogen, and the remaining mass in oxygen. determine its empirical formula. the formula mass of the unknown is independently determined to be 90.08 g/mol, determine the unknown’s molecular formula

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1


Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in car...
Questions


Mathematics, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20



English, 21.02.2021 04:20

Social Studies, 21.02.2021 04:20

Spanish, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20


History, 21.02.2021 04:20

Chemistry, 21.02.2021 04:20

Health, 21.02.2021 04:20

Social Studies, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20

Mathematics, 21.02.2021 04:20

English, 21.02.2021 04:20