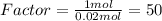Chemistry, 01.10.2019 18:20 poptropic9207
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅xh2o a sample of epsom salts with a mass of 4.93 g is heated to drive off the water of hydration. the mass of the sample after complete dehydration is 2.41 g. find the number of waters of hydration (x) in epsom salts

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Epsom salts is a hydrated ionic compound with the following formula: mgso4⋅xh2o a sample of epsom s...
Questions


Mathematics, 19.02.2020 00:02

Chemistry, 19.02.2020 00:02



History, 19.02.2020 00:02



Mathematics, 19.02.2020 00:02


Chemistry, 19.02.2020 00:02

Mathematics, 19.02.2020 00:02

Mathematics, 19.02.2020 00:02

Mathematics, 19.02.2020 00:02

History, 19.02.2020 00:02

History, 19.02.2020 00:02




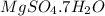
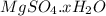 .After complete dehydration we have 2.41 g of
.After complete dehydration we have 2.41 g of 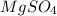 .
.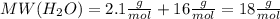
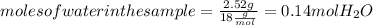
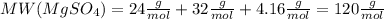
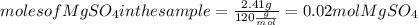
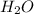 . Let´s find the multiplying factor to obtain the forula for 1 mol of
. Let´s find the multiplying factor to obtain the forula for 1 mol of