
Chemistry, 01.10.2019 19:00 kayyjayy3106
The work function of an element is the energy required to remove an electron from the surface of the solid element. the work function for lithium is 279.7 kj/mol (that is, it takes 279.7 kj of energy to remove one mole of electrons from one mole of li atoms on the surface of li metal). what is the maximum wavelength of light that can remove an electron from an atom on the surface of lithium metal?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these sequences lists the correct order for the creation of sedimentary rock from sediment? a. deposition, burial, compaction, cementation b. burial, deposition, compaction, cementation c. compaction, deposition, burial, cementation d. cementation, deposition, burial, compaction
Answers: 1

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
The work function of an element is the energy required to remove an electron from the surface of the...
Questions


Mathematics, 14.07.2020 02:01



Mathematics, 14.07.2020 02:01











Physics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

Biology, 14.07.2020 02:01

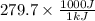
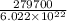
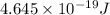
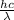
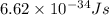
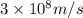
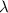 = wavelength
= wavelength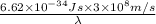
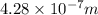
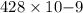 m
m

