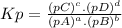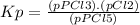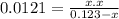Chemistry, 01.10.2019 19:00 sierravick123owr441
The equilibrium constant (k p) for the interconversion of pcl 5 and pcl 3 is 0.0121: pcl 5 (g) pcl 3 (g) cl 2 (g) a vessel is charged with pcl 5, giving an initial pressure of 0.123 atm. at equilibrium, the partial pressure of pcl 3 is atm.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1



Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
The equilibrium constant (k p) for the interconversion of pcl 5 and pcl 3 is 0.0121: pcl 5 (g) pcl...
Questions


Mathematics, 12.07.2019 10:40

History, 12.07.2019 10:40


History, 12.07.2019 10:40



English, 12.07.2019 10:40



History, 12.07.2019 10:40




Physics, 12.07.2019 10:40


Mathematics, 12.07.2019 10:40

Geography, 12.07.2019 10:40


![Kc = \frac{[C]^c.[D]^d}{[A]^a.[B]^b}](/tpl/images/0280/5110/4ea0c.png)