
Chemistry, 01.10.2019 20:30 anikabarnhart
Consider the following reaction representing the combustion of propane: + o2 → co2 + h2o a. balance the equation b. how many moles of oxygen are required to burn 1 mol of propane? c. how many grams of oxygen are required to burn 100 g of propane? d. at standard temperature and pressure, what volume of oxygen would be required to burn 100 g of propane? if air is 21 percent oxygen, what volume of air at stp would be required? e. at stp, what volume of carbon dioxide would be produced when 100 g of propane are burned? f. the standard enthalpy of propane is -103.8 kj/mol. find the gross heat released when 1 kg is burned.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
Consider the following reaction representing the combustion of propane: + o2 → co2 + h2o a. balanc...
Questions




Law, 13.12.2019 19:31

Mathematics, 13.12.2019 19:31

Computers and Technology, 13.12.2019 19:31

Mathematics, 13.12.2019 19:31

Mathematics, 13.12.2019 19:31

Mathematics, 13.12.2019 19:31



Advanced Placement (AP), 13.12.2019 19:31

Physics, 13.12.2019 19:31







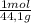 ×
×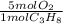 ×
×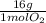 = 181 g of O₂
= 181 g of O₂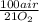 = 1210 L of air
= 1210 L of air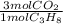 = 6,80 moles of CO₂
= 6,80 moles of CO₂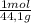 = 22,68 moles
= 22,68 moles

