
Chemistry, 01.10.2019 21:10 ofcitsnijah
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture is heated in the presence of excess cl2, all of the kbr is converted to kcl. if the total mass of kcl present after this reaction is 3.129 g, what percentage (by mass) of the original mixture was kbr? (hint: be sure that you understand why the mass of the sample has decreased. it may if you write an equation for the reaction that converted the kbr to kcl.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 22:00
8) warming your hands by a fire is an example if which heat transfer? a. conduction b. convection c. radiation d. none of these
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
Kcl and kbr are both ionic solids. a mixture of kcl and kbr has a mass of 3.595 g. when this mixture...
Questions

Mathematics, 13.08.2021 14:00


Mathematics, 13.08.2021 14:00

Chemistry, 13.08.2021 14:00


English, 13.08.2021 14:00



Biology, 13.08.2021 14:00




Mathematics, 13.08.2021 14:00

Mathematics, 13.08.2021 14:00


Chemistry, 13.08.2021 14:00

Advanced Placement (AP), 13.08.2021 14:00

English, 13.08.2021 14:00


Biology, 13.08.2021 14:00

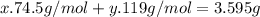
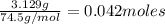
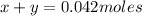
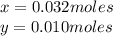
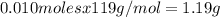
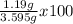 %
% 

