
Chemistry, 02.10.2019 01:00 reagriffis24
These are a mix (maybe) of ionic and covalent compounds: name these compounds. spell sulfide with the f not the ph. if the metal is a transition metal, you will need to use roman numerals in parentheses, no space between the metal and the parentheses, e. g. iron(iii) oxide covalent compounds should follow iupac naming methods. what is the name for pbr3? phosphorus tribromide what is the name for n2o? nitrous oxide what is the name for na2s? sodium sulfide what is the name for kmno4? potassium (iv) manganate

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
These are a mix (maybe) of ionic and covalent compounds: name these compounds. spell sulfide with t...
Questions


Social Studies, 25.11.2021 18:50

Mathematics, 25.11.2021 18:50

History, 25.11.2021 18:50

Biology, 25.11.2021 18:50

Chemistry, 25.11.2021 18:50

English, 25.11.2021 18:50

SAT, 25.11.2021 18:50

Mathematics, 25.11.2021 18:50

Mathematics, 25.11.2021 18:50





Mathematics, 25.11.2021 18:50

Social Studies, 25.11.2021 18:50



Mathematics, 25.11.2021 18:50

Computers and Technology, 25.11.2021 18:50

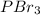 is phosphorous tribromide.
is phosphorous tribromide.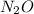 is dinitrogen oxide.
is dinitrogen oxide.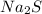 is sodium sulfide.
is sodium sulfide.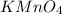 is potassium permanganate(VII).
is potassium permanganate(VII).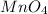 is permanganate.
is permanganate.

