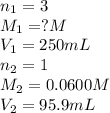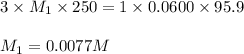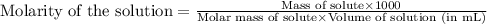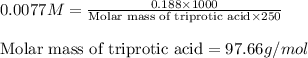Chemistry, 02.10.2019 02:00 Trevon0906
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask and dilutes to the mark with distilled water. he then titrates this solution with 0.0600 m naoh solutions. when the titration reaches the equivalence point, the chemist finds he has added 95.9 ml of naoh solution.
calculate the molar mass of the unknown acid.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1
You know the right answer?
An analytical chemist weighs out 0.188 g of an unknown triprotic acid into a 250 ml volumetric flask...
Questions




Mathematics, 18.04.2021 17:20

Mathematics, 18.04.2021 17:20


Mathematics, 18.04.2021 17:20

Biology, 18.04.2021 17:20

Mathematics, 18.04.2021 17:20



Health, 18.04.2021 17:20

Mathematics, 18.04.2021 17:20


Arts, 18.04.2021 17:20


Mathematics, 18.04.2021 17:20

Mathematics, 18.04.2021 17:20


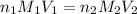
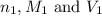 are the n-factor, molarity and volume of triprotic acid
are the n-factor, molarity and volume of triprotic acid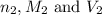 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.