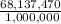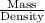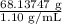Chemistry, 02.10.2019 20:00 ewalchloe5067920
Asolution used to chlorinate a home swimming pool contains 7% chlorine by mass. an ideal chlorine level for the pool is one part per million (1 ppm). (think of 1 ppm as being 1 g chlorine per million grams of water). if you assume densities of 1.10 g/ml for the chlorine solution and 1.00 g/ml for the swimming pool water, what volume of the chlorine solution, in liters, is required to produce a chlorine level of 1 ppm in an 18,000 gal swimming pool?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3


Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2
You know the right answer?
Asolution used to chlorinate a home swimming pool contains 7% chlorine by mass. an ideal chlorine le...
Questions

Mathematics, 30.01.2020 08:55

Health, 30.01.2020 08:55



English, 30.01.2020 08:55

Computers and Technology, 30.01.2020 08:55




Mathematics, 30.01.2020 08:55


Mathematics, 30.01.2020 08:55


History, 30.01.2020 08:55





Arts, 30.01.2020 08:55

Mathematics, 30.01.2020 08:55