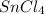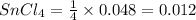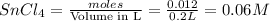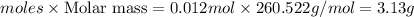Chemistry, 02.10.2019 19:30 domzilla115
Wenzhou prepares 200 ml of a solution of sncl4in which the concentration ofchloride ions is 0.240m. a) what is the molarity of the sncl4solution (i. e. what should the bottle be labeled)? b) what mass of sncl4did wenzhou use?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
Wenzhou prepares 200 ml of a solution of sncl4in which the concentration ofchloride ions is 0.240m....
Questions

History, 15.12.2021 03:10

Social Studies, 15.12.2021 03:10



Mathematics, 15.12.2021 03:10


English, 15.12.2021 03:10

Mathematics, 15.12.2021 03:10

Mathematics, 15.12.2021 03:10


English, 15.12.2021 03:10

Mathematics, 15.12.2021 03:10


Mathematics, 15.12.2021 03:10




Spanish, 15.12.2021 03:10

English, 15.12.2021 03:10

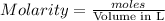
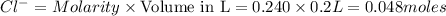
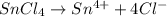
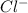 , there is 1 mole of
, there is 1 mole of