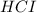Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn in water. (b) identify the brønsted-lowry conjugate acid-base pairs in the equation above. (c) write the chemical equation for the reaction of hcn with naoh. (d) write the chemical equation for dissociation of nacn in water. (e) write the chemical equation for the reaction of nacn and hci.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3
You know the right answer?
Hydrocyanic acid, hcn, is a weak acid. (a) write the chemical equation for the dissociation of hcn i...
Questions

Mathematics, 12.10.2019 23:30

History, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30


Mathematics, 12.10.2019 23:30



Advanced Placement (AP), 12.10.2019 23:30

History, 12.10.2019 23:30

History, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Health, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30



Biology, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

Mathematics, 12.10.2019 23:30

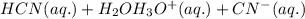
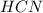 : acid
: acid 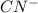 :conjugate base.
:conjugate base.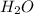 : base
: base 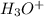 : conjugate acid.
: conjugate acid.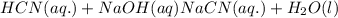
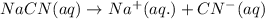
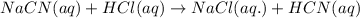
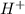 ions in their aqueous states.
ions in their aqueous states.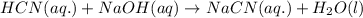
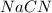 in water.
in water.