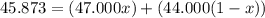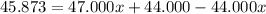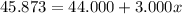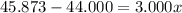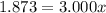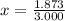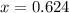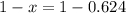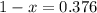Chemistry, 02.10.2019 20:10 BigGirlsTheBest
An element has only two naturally occurring isotopes. you know that isotope 1 has a mass of 44.000 amu and isotope 2 has a mass of 47.000 amu. if the average atomic mass is 45.873 amu, what are the natural abundances in percent for the two isotopes? 14.00m

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 23.06.2019 03:30
How many grams of sodium chloride are in 250ml of a 2.5m naci solution
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3
You know the right answer?
An element has only two naturally occurring isotopes. you know that isotope 1 has a mass of 44.000 a...
Questions

Business, 03.02.2021 06:20

Mathematics, 03.02.2021 06:20


Biology, 03.02.2021 06:20

History, 03.02.2021 06:20


History, 03.02.2021 06:20

Mathematics, 03.02.2021 06:20


Health, 03.02.2021 06:20





Mathematics, 03.02.2021 06:20

Biology, 03.02.2021 06:20

Mathematics, 03.02.2021 06:20

Mathematics, 03.02.2021 06:20