
Chemistry, 02.10.2019 20:10 21ghostrider21
Describe how you would prepare 100 ml of a 0.050 m sodium citrate tribasic buffer to a ph of 6. this method is accomplished by preparing a solution of a weak species base and then adding the appropriate strong species 0.5 m hcl to get to the desired ph. what amount of mass or volumes should you add?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Describe how you would prepare 100 ml of a 0.050 m sodium citrate tribasic buffer to a ph of 6. this...
Questions

Mathematics, 07.10.2019 11:50


Physics, 07.10.2019 11:50

English, 07.10.2019 11:50

History, 07.10.2019 11:50

Health, 07.10.2019 11:50

Mathematics, 07.10.2019 11:50



Biology, 07.10.2019 11:50

Chemistry, 07.10.2019 11:50


Mathematics, 07.10.2019 11:50


History, 07.10.2019 11:50

Social Studies, 07.10.2019 11:50

Business, 07.10.2019 11:50



![\frac{[A^{-}]}{[HA]}](/tpl/images/0284/0047/5980f.png)
![\frac{[CitrateTribasic]}{[CitrateDibasic]}](/tpl/images/0284/0047/f66e1.png)
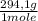 = 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-
= 1,4705 g of sodium citrate tribasic dihydrate -commercial reactant-

