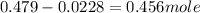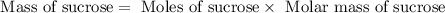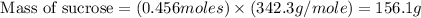The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order reaction with a rate constant of 1.8 x 10-45-1 at 25°c. determine the mass (g) of sucrose that is consumed when 2.15 l of a 0.223 m sucrose solution is allowed to react for 282 minutes. enter your answer as an integer. previous next not saved submit qu.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 16:00
Afuel has 30.43% nitrogen and 69.57% oxygen. find the molecular formula of the compound if it has a mass of 92 grams per mole a.no b.n2o4 c.no2 d.n4o8
Answers: 1

Chemistry, 23.06.2019 16:10
What type of reaction is shown below? check all that apply. agno3(aq) + nacl(aq) → nano3(aq) + agcl(s) i synthesis decomposition combustion i single replacement double replacement done
Answers: 2
You know the right answer?
The hydrolysis of sucrose (c12h22011) into glucose and fructose in acidic solution is a first-order...
Questions


Mathematics, 12.02.2021 09:50

Social Studies, 12.02.2021 09:50



English, 12.02.2021 09:50


English, 12.02.2021 09:50



Mathematics, 12.02.2021 09:50



Mathematics, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50


Business, 12.02.2021 09:50


Mathematics, 12.02.2021 09:50

Mathematics, 12.02.2021 09:50

![[C_t]=[C_o]e^{-kt}](/tpl/images/0284/1096/86a58.png)
![[C_t]](/tpl/images/0284/1096/81c01.png) = concentration of sucrose at time 't'
= concentration of sucrose at time 't'![[C_o]](/tpl/images/0284/1096/61f0a.png) = concentration of sucrose at time '0' = 0.223 M
= concentration of sucrose at time '0' = 0.223 M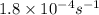
![[C_t]=(0.223)\times e^{-(1.8\times 10^{-4})\times (16920)}](/tpl/images/0284/1096/fccf3.png)
![[C_t]=0.0106M](/tpl/images/0284/1096/bb844.png)
![n_o=[C_o]\times V=0.223M\times 2.15L=0.479moles](/tpl/images/0284/1096/1c118.png)
![n_t=[C_t]\times V=0.0106M\times 2.15L=0.0228moles](/tpl/images/0284/1096/96015.png)