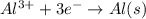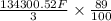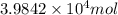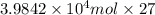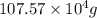Chemistry, 02.10.2019 21:00 genyjoannerubiera
Calculate the daily aluminum production of a 150,000 [a] aluminum cell that operates at a faradaic efficiency of 89%. the cell reaction is 2al2o3 + 3c → 4a1 + 3co2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2
You know the right answer?
Calculate the daily aluminum production of a 150,000 [a] aluminum cell that operates at a faradaic e...
Questions

English, 13.09.2021 14:30

History, 13.09.2021 14:30


English, 13.09.2021 14:30


Social Studies, 13.09.2021 14:30

Mathematics, 13.09.2021 14:40



English, 13.09.2021 14:40


Mathematics, 13.09.2021 14:40

History, 13.09.2021 14:40

SAT, 13.09.2021 14:40

Biology, 13.09.2021 14:40

Health, 13.09.2021 14:40



English, 13.09.2021 14:40

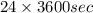
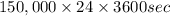
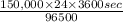
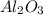 is +3.
is +3.