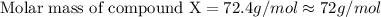When 5.42 g of a certain molecular compound x are dissolved in 80.0 g of formamide (nh, coh), the freezing point of the solution is measured to be -1.4 °c. calculate the molar mass of x. if you need any additional information on formamide, use only what you find in the aleks data resource. also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. one x 6 ?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 23.06.2019 05:50
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

Chemistry, 23.06.2019 06:30
Moving force of air flows from areas of high pressure to areas of low pressure true or false
Answers: 2
You know the right answer?
When 5.42 g of a certain molecular compound x are dissolved in 80.0 g of formamide (nh, coh), the fr...
Questions


Biology, 02.03.2021 20:50

Biology, 02.03.2021 20:50

English, 02.03.2021 20:50




Mathematics, 02.03.2021 20:50

Physics, 02.03.2021 20:50





Physics, 02.03.2021 20:50



Mathematics, 02.03.2021 20:50



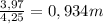
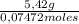 = 73 g/mol
= 73 g/mol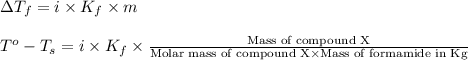
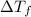 = change in freezing point
= change in freezing point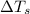 = freezing point of solution =
= freezing point of solution = 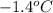
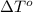 = freezing point of formamide =
= freezing point of formamide = 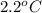
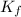 = freezing point constant for formamide =
= freezing point constant for formamide = 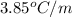
![[2.2-(-1.4)]^oC=1\times (3.85^oC/m)\times \frac{5.42g}{\text{Molar mass of compound X}\times 0.080kg}](/tpl/images/0284/0592/99dda.png)