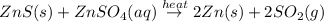Chemistry, 02.10.2019 20:30 Amandarobersonn
Zinc metal is produced by heating solid zinc sulfide with solid wine witte resulting in liquid zinc and sulfur dioxide gas write a balanced equation for the reaction using complete formulas for the compounds with phase labels (use the lowest possible coefficients. use the pull down boxes to specify states such as (aq) or (b). if a box is not needed, leave it blank.) submit answer try another version item attempts remaining

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3


Chemistry, 22.06.2019 21:30
Electromagnets coils of wire paper clips picked up 10 3 15 6 20 9 25 12 ms. owens' class was studying magnets. ms. owens showed her students how to make an electromagnet using a nail, a d-cell battery, and plastic coated wire. the students wrapped the wire around the nail and then attached the ends to the battery. when they were finished, they tested their magnets by investigating how many paperclips their magnets could pick up. they also tested whether they could increase the strength of their electromagnets by using more coils of wire. they recorded the class average of their results in the data table seen here. ms. owens asked her students to graph their data in a line graph. how should the students label the x-axis on their line graph? a) size of battery b) number of paper clips c) number of coils of wire d) strength of electromagnet
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Zinc metal is produced by heating solid zinc sulfide with solid wine witte resulting in liquid zinc...
Questions

Mathematics, 16.12.2020 17:40




English, 16.12.2020 17:40

Social Studies, 16.12.2020 17:40

Mathematics, 16.12.2020 17:40

Social Studies, 16.12.2020 17:40

Mathematics, 16.12.2020 17:40

Social Studies, 16.12.2020 17:40

Mathematics, 16.12.2020 17:40



Mathematics, 16.12.2020 17:40


Mathematics, 16.12.2020 17:40

English, 16.12.2020 17:40


Social Studies, 16.12.2020 17:40

Mathematics, 16.12.2020 17:40