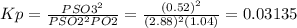Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 500 ml flask with 3.4 atm of sulfur dioxide gas and 1.3 atm of oxygen gas at 31. °c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 0.52 atm. calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answert significant digits. x i ? explanation

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions

Engineering, 01.06.2020 20:59

Arts, 01.06.2020 20:59

History, 01.06.2020 20:59

History, 01.06.2020 20:59

History, 01.06.2020 20:59


Mathematics, 01.06.2020 20:59


Mathematics, 01.06.2020 20:59

History, 01.06.2020 20:59

Mathematics, 01.06.2020 20:59






History, 01.06.2020 20:59

Mathematics, 01.06.2020 20:59

Physics, 01.06.2020 20:59