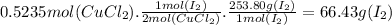Chemistry, 02.10.2019 21:10 blachaze8729
12 is produced by the reaction of 0.5235 mol of cucl2 according to the following equation: 2cucl2 +4k1—2cul + 4kci + 12. what is the mass of the la produced? 66.43 g 33.22 g 132.9 g

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
12 is produced by the reaction of 0.5235 mol of cucl2 according to the following equation: 2cucl2 +...
Questions


Mathematics, 27.01.2021 08:50

History, 27.01.2021 08:50

Mathematics, 27.01.2021 08:50




Mathematics, 27.01.2021 08:50


Mathematics, 27.01.2021 08:50

History, 27.01.2021 08:50

History, 27.01.2021 08:50



Mathematics, 27.01.2021 08:50


Mathematics, 27.01.2021 08:50


English, 27.01.2021 08:50

English, 27.01.2021 08:50