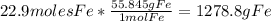Chemistry, 02.10.2019 21:20 fancycar14
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the balanced equation for this reaction? (use the lowest possible coefficients. omit states of matter.) b. what number of moles of iron reacts with 17.15 mol of oxygen from the air? mol c. what mass of iron is required to react with 17.15 mol of oxygen? mass =

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Iron(iii) oxide, fe2o3, is a result of the reaction of iron with the oxygen in air. a. what is the b...
Questions


Mathematics, 16.12.2019 04:31







Mathematics, 16.12.2019 04:31

History, 16.12.2019 04:31


Mathematics, 16.12.2019 04:31



Mathematics, 16.12.2019 04:31





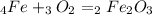
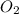
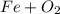
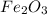 , so you should write the product:
, so you should write the product: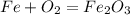
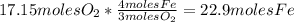
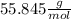 , so:
, so: