
Chemistry, 02.10.2019 22:00 hunterwilliams375
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benzene, c6h6? . the vapor pressure of pure benzene at 23 oc is 86.0 mm hg; the vapor pressure of naphthalene can be neglected. calculate the vapor-pressure lowering of the solution.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
What is the vapor pressure at 23 oc of a solution of 1.20 g of naphthalene, c10h8, in 25.6 g of benz...
Questions


Biology, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00


Mathematics, 03.11.2020 01:00



English, 03.11.2020 01:00




Computers and Technology, 03.11.2020 01:00


Mathematics, 03.11.2020 01:00

Biology, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

English, 03.11.2020 01:00

Mathematics, 03.11.2020 01:00

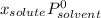 (1)
(1)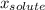 is molar fraction of solute
is molar fraction of solute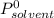 is the capor pressure of the pure solvent (86,0 mmHg)
is the capor pressure of the pure solvent (86,0 mmHg)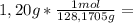 9,36x10⁻³ moles solute
9,36x10⁻³ moles solute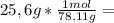 0,328 moles solvent
0,328 moles solvent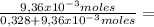 0,0278
0,0278

