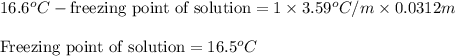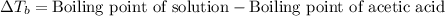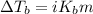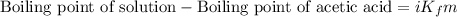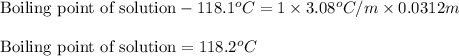Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3
You know the right answer?
Asolution was prepared by dissolving 0.800 g of sulfur s8, in 100.0 g of acetic acid, hc2h3o2. calcu...
Questions



Mathematics, 07.12.2020 18:00

Computers and Technology, 07.12.2020 18:00

Mathematics, 07.12.2020 18:00



Physics, 07.12.2020 18:00

Mathematics, 07.12.2020 18:00

Mathematics, 07.12.2020 18:00




Chemistry, 07.12.2020 18:00

Biology, 07.12.2020 18:00


Mathematics, 07.12.2020 18:00



Arts, 07.12.2020 18:00

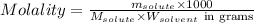
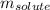 = Given mass of solute
= Given mass of solute 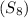 = 0.800 g
= 0.800 g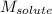 = Molar mass of solute
= Molar mass of solute  = 256.52 g/mol
= 256.52 g/mol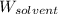 = Mass of solvent (acetic acid) = 100.0 g
= Mass of solvent (acetic acid) = 100.0 g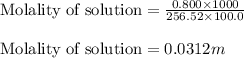
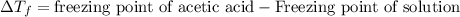
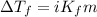
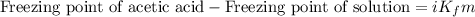
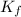 = molal freezing point depression constant = 3.59°C/m
= molal freezing point depression constant = 3.59°C/m