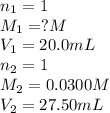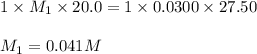Chemistry, 02.10.2019 22:00 art3misdiangelo
20.0 ml sample of hcl, ist te of hel is titrated with 0.0300m koh solution. the volume of potassium droxide required is 27.50ml. what is the molarity of hci solution? +koh → kci+h2o

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
20.0 ml sample of hcl, ist te of hel is titrated with 0.0300m koh solution. the volume of potassium...
Questions

Mathematics, 17.06.2021 09:50

Social Studies, 17.06.2021 09:50


Physics, 17.06.2021 09:50

English, 17.06.2021 09:50



Mathematics, 17.06.2021 09:50


Mathematics, 17.06.2021 09:50


Mathematics, 17.06.2021 09:50


Mathematics, 17.06.2021 09:50

Geography, 17.06.2021 09:50

Physics, 17.06.2021 09:50

Physics, 17.06.2021 09:50


Mathematics, 17.06.2021 09:50

Mathematics, 17.06.2021 09:50

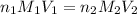
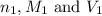 are the n-factor, molarity and volume of acid which is HCl
are the n-factor, molarity and volume of acid which is HCl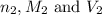 are the n-factor, molarity and volume of base which is KOH
are the n-factor, molarity and volume of base which is KOH