

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
a solution of 0.170 mol/l ion strength is prepared using nano3 (solid) and na2so4 (solid). calculate...
Questions

Mathematics, 17.12.2020 01:30

Mathematics, 17.12.2020 01:30

English, 17.12.2020 01:30

Biology, 17.12.2020 01:30

Computers and Technology, 17.12.2020 01:30


English, 17.12.2020 01:30


Mathematics, 17.12.2020 01:30

Mathematics, 17.12.2020 01:30

English, 17.12.2020 01:30





Social Studies, 17.12.2020 01:30



Arts, 17.12.2020 01:30

Mathematics, 17.12.2020 01:30

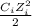 Where Ci and Zi are concentration and charge of each ion in solution. Thus:
Where Ci and Zi are concentration and charge of each ion in solution. Thus: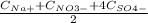 = 0,170 mol/L
= 0,170 mol/L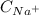 = 0,130 mol/L you can obtain:
= 0,130 mol/L you can obtain: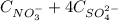 (1)
(1)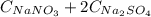 , thus:
, thus: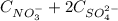 (2)
(2)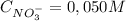
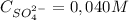
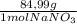 = 1,06 g of NaNO₃
= 1,06 g of NaNO₃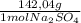 = 1,42 g of Na₂SO₄
= 1,42 g of Na₂SO₄

