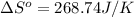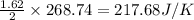Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3
You know the right answer?
Consider the reaction: 2brf3(g) --> br2(g) + 3f2(g)
using standard absolute entropi...
using standard absolute entropi...
Questions


Social Studies, 11.02.2020 04:32

Mathematics, 11.02.2020 04:32

History, 11.02.2020 04:33

Social Studies, 11.02.2020 04:33



Mathematics, 11.02.2020 04:33


Social Studies, 11.02.2020 04:33

Mathematics, 11.02.2020 04:33

Mathematics, 11.02.2020 04:33




Mathematics, 11.02.2020 04:35

Social Studies, 11.02.2020 04:35


Mathematics, 11.02.2020 04:36

Mathematics, 11.02.2020 04:36

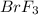 reacts at standard condition is 217.68 J/K
reacts at standard condition is 217.68 J/K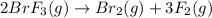
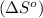 is:
is: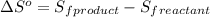
![\Delta S^o=[n_{Br_2}\times \Delta S_f^0_{(Br_2)}+n_{F_2}\times \Delta S_f^0_{(F_2)}]-[n_{BrF_3}\times \Delta S_f^0_{(BrF_3)}]](/tpl/images/0284/2284/c6a07.png)
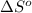 = entropy change of reaction = ?
= entropy change of reaction = ?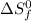 = standard entropy of formation
= standard entropy of formation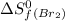 = 245.463 J/mol.K
= 245.463 J/mol.K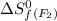 = 202.78 J/mol.K
= 202.78 J/mol.K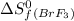 = 292.53 J/mol.K
= 292.53 J/mol.K![\Delta S^o=[1mole\times (245.463J/K.mole)+3mole\times (202.78J/K.mole)}]-[2mole\times (292.53J/K.mole)]](/tpl/images/0284/2284/4a0f6.png)