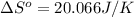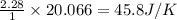Chemistry, 02.10.2019 21:30 dontworry48
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies at 298k, calculate the entropy change for the system when 2.28 moles of h2(g)react at standard conditions.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1


Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Consider the reaction: h2(g) + cl2(g) --> 2hcl(g)
using standard absolute entropies...
using standard absolute entropies...
Questions

Mathematics, 17.08.2020 17:01

History, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01

Computers and Technology, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01


Chemistry, 17.08.2020 17:01

Biology, 17.08.2020 17:01

Spanish, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01




Mathematics, 17.08.2020 17:01


Mathematics, 17.08.2020 17:01


 reacts at standard condition is 45.8 J/K
reacts at standard condition is 45.8 J/K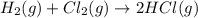
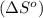 is:
is: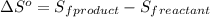
![\Delta S^o=[n_{HCl}\times \Delta S_f^0_{(HCl)}]-[n_{H_2}\times \Delta S_f^0_{(H_2)}+n_{Cl_2}\times \Delta S_f^0_{(Cl_2)}]](/tpl/images/0284/2286/d7d45.png)
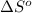 = entropy change of reaction = ?
= entropy change of reaction = ?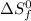 = standard entropy of formation
= standard entropy of formation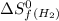 = 130.684 J/mol.K
= 130.684 J/mol.K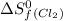 = 223.066 J/mol.K
= 223.066 J/mol.K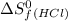 = 186.908 J/mol.K
= 186.908 J/mol.K![\Delta S^o=[2mole\times (186.908J/K.mole)]-[1mole\times (130.684J/K.mole)+1mole\times (223.066J/K.mole)}]](/tpl/images/0284/2286/55b6e.png)