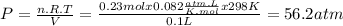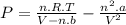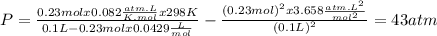Chemistry, 02.10.2019 21:30 allyssaharrisooy50au
How reliable is the perfect gas law in comparison with the van der waals equation? calculate the difference in pressure of 10.00g of co2 confined to a container of volume 100cm3 at 25 degree centigrade between treating it as a perfect gas and a van der waals gas

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 14:00
Comparing john newland’s octaves with the modern periodic table, which 5 elements have been discovered between hydrogen and iron since newland’s time?
Answers: 3


Chemistry, 23.06.2019 16:30
Match the words in the left column to the appropriate blanks in the sentences on the right. (a) this is the law of definite proportions: all samples of a given compound, regardless of their source or how they were prepared, have proportions of their constituent elements. (b) this is the law of conservation of mass: in a reaction, matter is neither created nor destroyed. (c) this is the law of multiple (c) this is the law of multiple blank: when two elements form two different compounds, the masses of element b that combine with 1 g of element a can be expressed as a ratio of small whole-numbers. in this example, the ratio of \rm o from hydrogen peroxide to \rm o from water = 16: 8 \to 2: 1, a small whole-number ratio.: when two elements form two different compounds, the masses of element b that combine with 1 g of element a can be expressed as a ratio of small whole-numbers. in this example, the ratio of o from hydrogen peroxide to o from water = 16: 8 → 2: 1, a small whole-number ratio.
Answers: 1
You know the right answer?
How reliable is the perfect gas law in comparison with the van der waals equation? calculate the dif...
Questions



English, 25.08.2019 16:10


Mathematics, 25.08.2019 16:10

Mathematics, 25.08.2019 16:10



Biology, 25.08.2019 16:10

Physics, 25.08.2019 16:10

Biology, 25.08.2019 16:10







Biology, 25.08.2019 16:10