Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 22.06.2019 23:00
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
For a certain chemical reaction, the standard gibbs free energy of reaction at 10.0 °c is 149. kj. c...
Questions



Mathematics, 27.08.2019 00:30


Chemistry, 27.08.2019 00:30

Mathematics, 27.08.2019 00:30





English, 27.08.2019 00:30

Biology, 27.08.2019 00:30

Mathematics, 27.08.2019 00:30

Biology, 27.08.2019 00:30

Mathematics, 27.08.2019 00:30



Geography, 27.08.2019 00:30


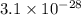
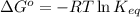
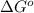 = standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )
= standard Gibbs free energy = 149. kJ/mol = 149000 J/mol (Conversion factor: 1 kJ = 1000 J )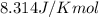
![15^oC=[273+15]K=283K](/tpl/images/0284/3257/82fe2.png)
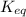 = equilibrium constant at 10°C = ?
= equilibrium constant at 10°C = ?